INVESTMENT
Implant Could End Eye Drops: A New Era in Glaucoma Treatment
US start-up advances device replacing daily drops with sustained-release therapy
6 Jun 2025
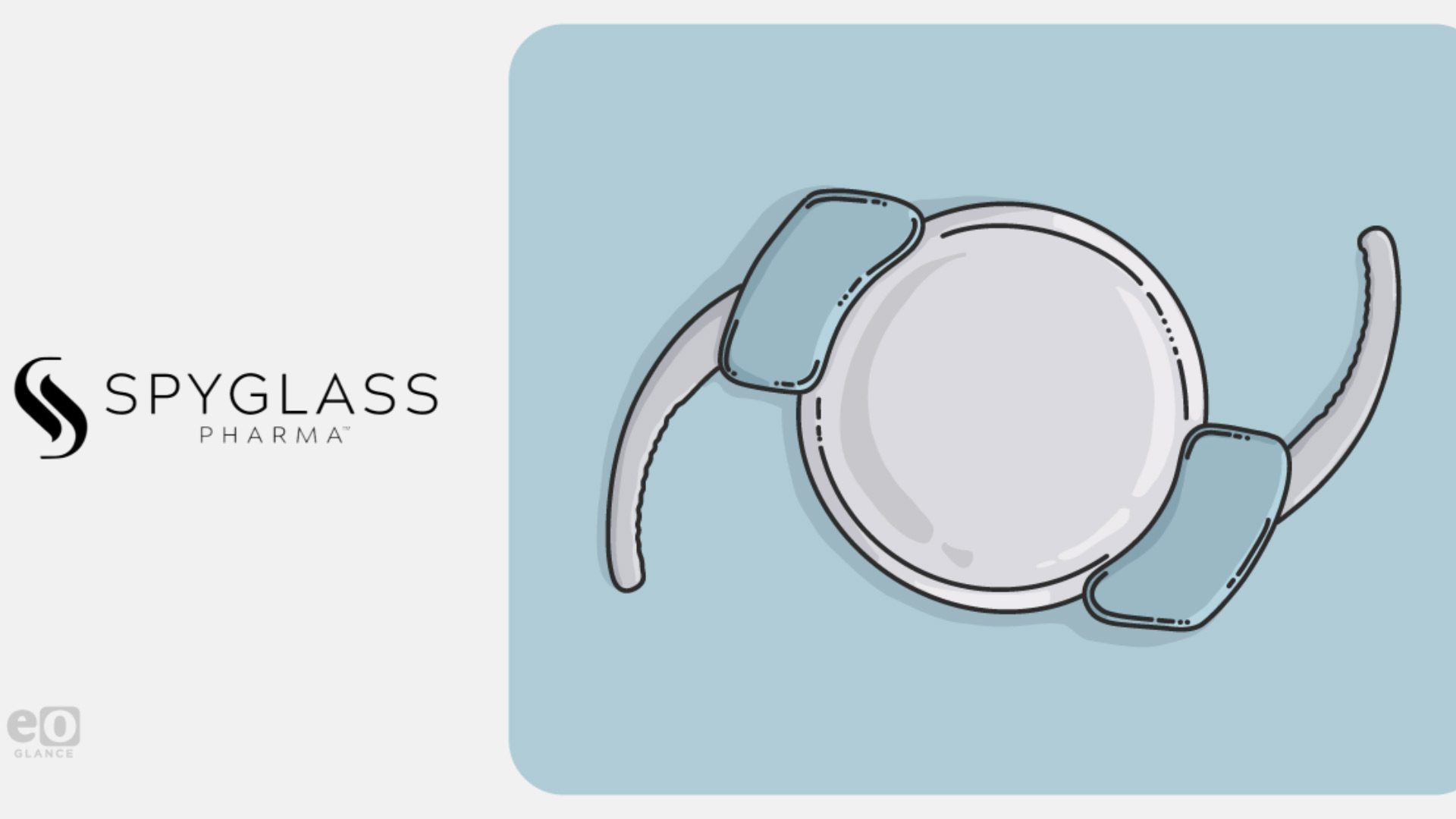
SpyGlass Pharma has secured funding to begin Phase III trials of an implant designed to treat glaucoma without the need for daily eye drops, in a development that could alter long-term care for chronic eye disease.
The US-based company attracted backing from a group of healthcare investors including RA Capital, NEA, Sands Capital and Gilde Healthcare. The capital will support the late-stage testing of a micro-implant that delivers intraocular pressure-lowering drugs directly into the eye over several months.
The device targets primary open-angle glaucoma, a leading cause of irreversible blindness. SpyGlass aims to improve treatment by addressing a long-standing problem in ophthalmology: poor adherence to daily medication routines. By automating drug delivery, the implant seeks to improve outcomes while reducing clinic visits.
“The goal is to ease the treatment burden on patients and physicians alike,” the company said. The firm’s backers view it as part of a broader shift in medicine toward long-acting, hands-free therapies. Similar approaches are gaining traction in other areas of chronic care, including diabetes and mental health.
While the technology promises greater convenience and reliability, it must still demonstrate long-term safety and clinical benefit. Previous attempts at ocular implants have faced challenges related to surgical complexity and patient tolerance. SpyGlass says its device is designed for straightforward insertion during cataract surgery, one of the most common procedures worldwide.
The trials come at a time when the pharmaceutical industry is paying close attention to sustained-release delivery systems. If successful, the SpyGlass implant could offer a new model for managing chronic illness, removing the need for daily intervention and enabling more consistent drug exposure.
The company has not disclosed a timeline for potential regulatory submission. However, if Phase III results are positive, the implant could enter the US market within the next few years.
Latest News
13 Feb 2026
Core-Shell LNP Study Signals Promise, Not Proof12 Feb 2026
FDA Aligns Device Rules With Global Standard11 Feb 2026
Smart Microneedle Patches Reshape Drug Delivery10 Feb 2026
Integrated CDMO Alliance Targets Oral Drug Timelines
Related News
RESEARCH
13 Feb 2026
Core-Shell LNP Study Signals Promise, Not Proof

REGULATORY
12 Feb 2026
FDA Aligns Device Rules With Global Standard
INNOVATION
11 Feb 2026
Smart Microneedle Patches Reshape Drug Delivery
SUBSCRIBE FOR UPDATES
By submitting, you agree to receive email communications from the event organizers, including upcoming promotions and discounted tickets, news, and access to related events.